You have terrible heartburn and you reach for an antacid. You know it stops the burning, but you might not know why. This confusion could make you unsure if you are using the right product.
An antacid is a base. Its job is to perform a chemical reaction called neutralization. It uses its basic (or alkaline) properties to counteract the strong hydrochloric acid in your stomach, raising the pH and providing relief from the burning sensation of heartburn.

From my perspective managing production at our aluminum hydroxide plant, this question gets to the very heart of what our product does. Our entire business is based on a simple chemical principle: a base neutralizes an acid. When I speak with clients like Mr. Park in Korea, who distributes pharmaceutical ingredients, he needs to trust that we understand this science perfectly. The pure, white powder we make is specifically designed to be an effective, safe base for use in antacid1 medications. Understanding this is key to understanding its value.
Is an antacid an acid or base?
The name "antacid" has the word "acid" in it, which can be confusing. You might wonder if it contains some special type of acid that fights the acid in your stomach.
An antacid is a base, not an acid. The "ant-" prefix means "against" or "opposite." So, an "ant-acid" is a substance that works against acid. Its basic nature is what allows it to neutralize stomach acid and relieve heartburn.

This is one of the most fundamental concepts in chemistry. Everything can be measured on a pH scale2, which runs from 0 to 14. A pH of 7 is neutral, like pure water. Anything below 7 is an acid, and anything above 7 is a base. The acid in your stomach is extremely strong, with a very low pH of around 1.5 to 3.5. An antacid is a chemical base. Our aluminum hydroxide, for instance, is a weak base. When you take it, it raises the stomach’s pH. The goal isn’t to make your stomach completely neutral, but to raise the pH just enough to stop the burning, usually to a level above 3.5. This controlled neutralization3 is why specific bases like aluminum hydroxide and calcium carbonate are used. They are strong enough to do the job but gentle enough not to cause a drastic chemical change in the stomach.
pH Scale Basics
| Substance | pH Level (Approximate) | Classification |
|---|---|---|
| Stomach Acid (HCl) | 1.5 – 3.5 | Strong Acid |
| Black Coffee | 5.0 | Weak Acid |
| Pure Water | 7.0 | Neutral |
| Antacids | 8.0 – 10.0 | Base |
| Soapy Water | 12.0 | Strong Base |
Are antacids an acid or alkaline?
You hear the words "base" and "alkaline" used to describe antacids. Are they different things? This can add another layer of confusion when you are just trying to understand how they work.
Antacids are alkaline. The words "alkaline" and "base" mean almost the same thing in this context. An alkali is a specific type of base that can dissolve in water. Since antacids work in the watery environment of your stomach, they are alkaline bases.

In my work, precision matters, but for this topic, you can think of base and alkaline4 as the same thing. The important idea is what happens when the alkaline antacid meets the stomach acid. It triggers a neutralization reaction. This is the simple and powerful chemistry at the center of our business. The general formula is: Acid + Base → Salt + Water. When our aluminum hydroxide (a base) reacts with hydrochloric acid5 in the stomach, it looks like this: Al(OH)₃ + 3HCl → AlCl₃ + 3H₂O. You start with a harmful, burning acid. After the reaction, you are left with aluminum chloride (a harmless salt in this context) and water. The acid is gone. It has been neutralized. This is not magic; it is straightforward chemistry. This reaction is why our customers buy our product.
What type of acid is an antacid?
Thinking an antacid might be a special kind of acid is a common mistake. After all, it seems logical that you might fight fire with a different kind of fire.
This is a trick question: an antacid is not any type of acid at all. It is chemically the opposite of an acid. An antacid is always a base. Its entire purpose is to have the opposite properties of an acid so it can neutralize it effectively.
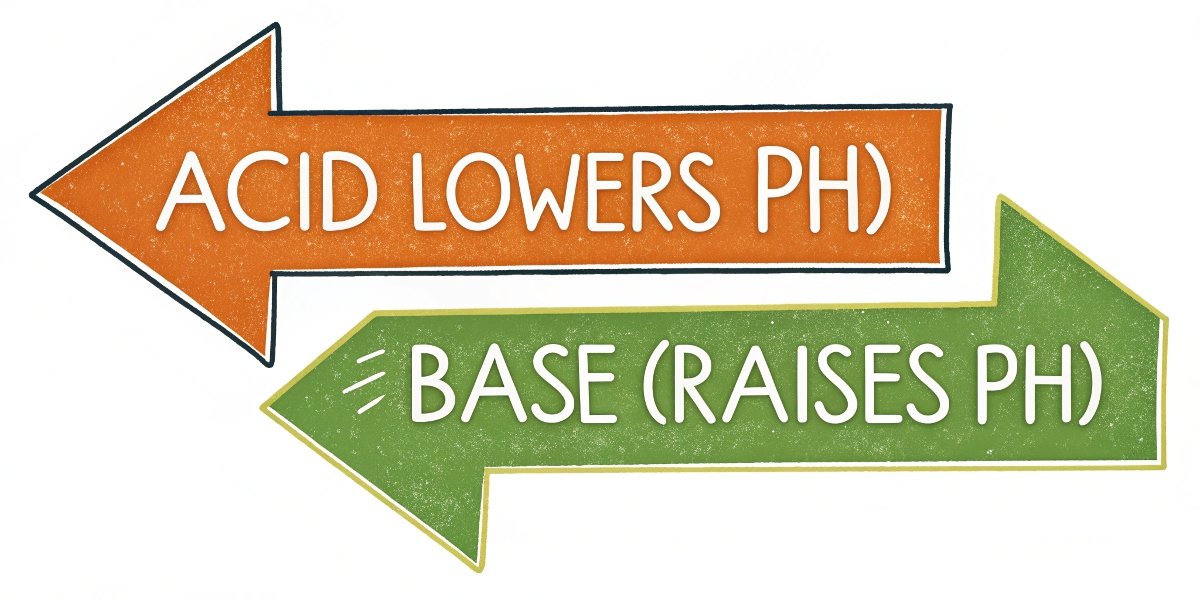
Let’s make the distinction very clear. Acids and bases have completely opposite chemical behaviors. An acid is a substance that releases hydrogen ions (H⁺) in a solution, which makes the solution more acidic and lowers its pH. A base is a substance that accepts those hydrogen ions, removing them from the solution and raising the pH. Our product, aluminum hydroxide, Al(OH)₃, works by releasing hydroxide ions (OH⁻). These hydroxide ions6 then react with the acid’s hydrogen ions (H⁺) to form water (H₂O), which is neutral. This is the definition of neutralization. It cannot be an acid because its chemical function is to do the exact opposite of what an acid does. It’s not about fighting acid with a "weaker acid," but about canceling it out with its chemical counterpart: a base.
Acid vs. Base Comparison
| Property | Acid | Base (Antacid) |
|---|---|---|
| Taste | Sour (e.g., lemon juice) | Bitter, chalky (e.g., baking soda) |
| Effect on pH | Lowers pH (makes it < 7) | Raises pH (makes it > 7) |
| Chemical Action | Donates hydrogen ions (H⁺) | Accepts hydrogen ions (H⁺) |
| Example | Hydrochloric Acid (HCl) in stomach | Aluminum Hydroxide (Al(OH)₃) |
What are antacids classified as?
When you look at a medicine bottle, you see technical terms. How do scientists and doctors officially classify these products? Knowing this helps you understand their place in medicine.
Chemically, antacids are classified as inorganic bases. In medicine, they are classified as acid neutralizers. They are part of a larger group of drugs for dyspepsia but are distinct from drugs that reduce acid production, like H2 blockers or proton pump inhibitors (PPIs).

This classification is important for a distributor like Mr. Park. He needs to know exactly where our product fits in the market.
- Chemical Classification: The active ingredients are simple inorganic compounds. The most common are aluminum hydroxide, magnesium hydroxide, calcium carbonate, and sodium bicarbonate. All are bases.
- Medical Classification: Their function is "acid neutralization." They work directly on the acid already present in the stomach, offering fast, short-term relief. This makes them different from more advanced medications. H2 blockers7 (like Zantac) and PPIs (like Prilosec) work much more slowly. They don’t neutralize existing acid. Instead, they work on a cellular level to signal your stomach to produce less acid in the first place. An antacid is like a firefighter putting out an existing fire. A PPI is like a fire prevention system that stops the fire from starting. Both are useful, but they have very different roles and speeds. Our product is for the "firefighter" role: fast, direct relief.
Conclusion
Antacids are bases, also known as alkaline compounds. They are chemically the opposite of acids and work by performing a simple neutralization reaction to provide fast relief from stomach acid.
-
Understanding what an antacid is can clarify its role in treating heartburn and its chemical properties. ↩
-
Understanding the pH scale is crucial for grasping how antacids affect stomach acidity. ↩
-
Exploring neutralization reactions can deepen your understanding of how antacids relieve heartburn. ↩
-
Exploring the concept of alkalinity can clarify how antacids function in the body. ↩
-
Learning about hydrochloric acid can help you understand the environment antacids are working in. ↩
-
Learning about hydroxide ions can clarify their importance in the neutralization process. ↩
-
Comparing H2 blockers with antacids can clarify their different roles in managing stomach acid. ↩
You may also be interested in:

What protective measures should be taken when handling aluminum hydroxide powder in a factory?
Are you worried about worker safety when handling fine powders? Aluminum hydroxide is generally safe, but mishandling its dust can lead to serious respiratory issues and even create explosive conditions.

What Key Technical Indicators Should Be Considered When Purchasing Aluminum Hydroxide?
Are you struggling to find the right aluminum hydroxide for your needs? You might be paying for features you don’t even require. Let’s simplify the technical details. When buying aluminum

What are the market trends for aluminum hydroxide in 2026?
Are you planning your 2026 raw material purchases? Volatile markets make it hard. I will share insights from the factory floor to help you make better decisions for your business.

Is aluminum hydroxide toxic and is it harmful to the human body?
Worried about the word "aluminum" in your products? It sounds scary and can make you question its safety. I’m here to clear up the confusion with simple facts. Aluminum hydroxide
What are the side effects of long-term use of stomach medications containing aluminum hydroxide?
Struggling with constant heartburn? Reaching for that stomach medicine might seem like the only option. But what if that relief comes with hidden long-term costs? The most common side effect

Does ingesting aluminum (such as through aluminum hydroxide) cause Alzheimer's disease?
Are you worried that everyday products containing aluminum might be harmful? This fear connects to serious health concerns like Alzheimer’s, making you question what is safe. Let’s look closely. Based
Why do some vaccines contain aluminum hydroxide (aluminum adjuvant)?
Confused about vaccine ingredients? Seeing aluminum hydroxide listed can be unsettling. I’ll explain its crucial role and why it’s there to help your body build strong immunity. Aluminum hydroxide acts

Is the aluminum adjuvant in vaccines safe for infants or adults?
You read about vaccine ingredients and worry. The word "aluminum" sounds scary, especially when talking about babies. But understanding the facts can give you peace of mind. Yes, the aluminum

What environmental problems are generated during the production of aluminum hydroxide?
Worried about environmental compliance in your supply chain? Sourcing from China can be complex. You need a reliable, eco-conscious partner for your aluminum hydroxide needs. The main environmental issue is

Where are China's main aluminum hydroxide production areas?
Are you struggling to find the right aluminum hydroxide supplier in China? This confusion can lead to higher costs and unstable quality, which hurts your business and your reputation. China’s